Background: As the population ages, the prevalence of hematologic malignancies in older adults will continue to increase. Older adults, especially older than 75 years, are routinely underrepresented in clinical trials evaluating hematologic malignancies, largely due to access and logistics, comorbidities, toxicity concerns, and eligibility restrictions. Given the recent approvals of multiple CAR T-cell therapies for patients with hematologic malignancies, we evaluated the rates of enrollment of older adults (age ≥ 65 years) in registrational trials of CAR T-cell therapies submitted to the U.S. Food and Drug Administration (FDA).
Methods: We conducted a pooled analysis of the registrational trials submitted to the FDA between 2017 and 2021 that supported approvals of autologous CAR T-cell therapies in patients with hematologic malignancies. Datasets submitted with the biologic license applications were pooled and analyzed descriptively for demographics, disease characteristics, and efficacy outcomes. Patients between the ages of 65 and 74 and age 75 and above were analyzed separately.
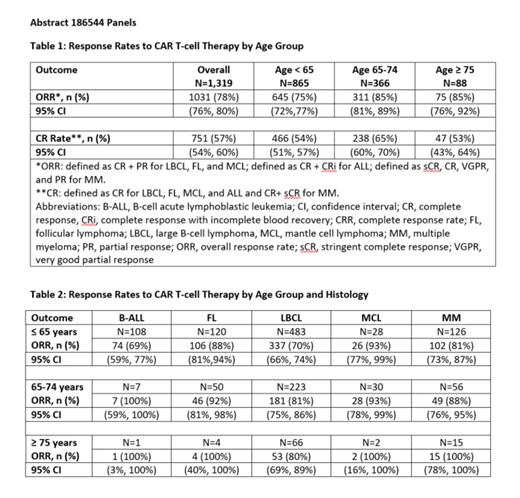
Results: Thirteen registrational trials evaluating six CAR T-cell products in 1,319 patients with hematologic malignancies were submitted to FDA. The median age at enrollment was 60 years (range: 3 to 86). In total, 7% (88) of patients were 75 years and older, 28% (366) were between the ages of 65 and 74 years, and 66% (865) were under the age of 65 years. The hematologic malignancies included large B-cell lymphoma (59%), multiple myeloma (15%), follicular lymphoma (13%), B-cell acute lymphoblastic leukemia (9%), and mantle cell lymphoma (5%). Response rates by age group and histology are shown in Table 1 and Table 2.
Conclusions: Older adults, particularly those aged 75 years or older, were underrepresented in the trials supporting FDA approval of CAR T-cell therapies considering the predominant hematologic malignancies included in this pooled analysis and the median age of diagnosis of these diseases in the U.S. population. Although clinically meaningful efficacy was achieved across age groups and diseases, more data are needed to determine the impact of age on efficacy outcomes in specific malignancies. In this analysis, data were limited among patients 75 years and older in all histologies except large B cell lymphoma. Work to evaluate the impact of age on safety and tolerability outcomes is ongoing. To improve accrual, greater efforts to understand and reduce barriers to clinical trial enrollment for older adults are needed, especially given the expanding use of CAR T-cell therapies and their toxicity profiles.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal